Many of us on Earth dream of temporarily joining the Expedition 1 crew aboard the International Space Station (ISS). Imagine floating effortlessly between modules, gazing down at Earth from a stunning 350 kilometers above the surface… For countless space enthusiasts, this is the ultimate dream.
But before you get too excited, it’s important to understand the reality of life aboard the ISS. While the view and weightlessness are awe-inspiring, living in space comes with its own set of challenges—tight spaces, constant work, and… what’s that smell? It’s likely the result of outgassing from a scientific experiment or, perhaps, a more personal source: a crewmate.
With 3 to 7 astronauts living and working in a confined area aboard the Space Station, air management becomes absolutely crucial.
The ISS life support systems are designed to provide oxygen and remove carbon dioxide from the cabin’s atmosphere, but that’s just the beginning. The air also needs to be kept free from trace gases like ammonia and acetone, which we all exhale in small amounts. Moreover, vapors from scientific experiments could pose a risk if they react with other elements in the air supply in unpredictable ways.
So, while space air is undeniably scarce, managing it is one of the significant challenges for ISS life support engineers.
In this second article of our series on the practical challenges of space living, Science@NASA explores how the ISS ensures that its astronauts have the breath of life.
Making Oxygen from Water
Humans can only survive for a few minutes without oxygen, and even low levels can lead to fatigue or blackouts.
To ensure the crew’s safety, the ISS is equipped with multiple redundant systems to guarantee a continuous supply of this essential gas.
“The primary source of oxygen will be water electrolysis, supplemented by O2 stored in pressurized tanks,” explained Jay Perry, an aerospace engineer at NASA’s Marshall Space Flight Center, who is working on the Environmental Control and Life Support Systems (ECLSS) project. Engineers at Marshall, the Johnson Space Center, and other NASA locations are focused on developing, testing, and improving the primary life support systems for the ISS.
Most of the oxygen aboard the ISS will be produced through a process called “electrolysis.” This process uses electricity generated by the ISS’s solar panels to break down water into hydrogen and oxygen gases.

The ISS’s first crew aboard the Space Station, Bill Shepherd, Sergei Krikalev, and Yuri Gidzenko. During their four-month mission, they will rely on the Station’s systems to provide breathable air.
Water molecules are made up of two hydrogen atoms and one oxygen atom. By passing an electrical current through water, the atoms separate, with hydrogen (H2) and oxygen (O2) forming as separate gases.
While the oxygen we breathe on Earth also comes from splitting water, it occurs through a natural process, not a mechanical one. Plants, algae, cyanobacteria, and phytoplankton all use water splitting during photosynthesis, a process in which sunlight, carbon dioxide, and water are transformed into sugars. The hydrogen is used in the formation of sugars, and the oxygen is released into the atmosphere.
“Ultimately, it would be ideal if we could rely on plants to produce oxygen for us,” said Monsi Roman, chief microbiologist for the ECLSS project at MSFC. “The added benefit of plants is that they also provide food.”
However, Perry noted, “Chemical and mechanical systems are much more compact, less labor-intensive, and more reliable than a plant-based approach. Right now, plant-based life support systems are still in the early stages of research and demonstration, and there are many challenges to overcome before they can become viable.”
The hydrogen left over from the electrolysis process will initially be vented into space. NASA engineers have designed the ECLSS hardware with space to accommodate a future machine that will combine this hydrogen with excess carbon dioxide from the air, creating water and methane. The water will help replenish the supply used to produce oxygen, while the methane will be released into space.
“We aim to close the loop completely, where everything is reused,” Roman explained. Various uses for the methane are being explored, including venting it to help provide thrust to maintain the ISS’s orbit.
Currently, Perry said, “All vented gases are designed to be non-propulsive.”
The ISS will also have large oxygen tanks mounted on the outside of the airlock module. These tanks will serve as the primary oxygen source for the U.S. segment of the ISS until Node 3’s life support systems are operational in 2005. Afterward, the tanks will act as a backup supply.
Last week, while waiting for the activation of a water electrolysis machine on the Zvezda Service Module, the crew relied on “perchlorate candles” for oxygen. These candles generate O2 through chemical reactions within a metal canister.
“You have a metal canister filled with perchlorate material,” Perry explained. “The canister is inserted into a reactor, and then an igniter pin is pulled. Once the reaction begins, it continues to burn until the material is used up.” Each canister provides enough oxygen for one person for one day.
“It’s essentially the same technology used in commercial aircraft,” he continued. “When the oxygen mask drops, pulling on it activates the igniter pin. That’s what starts the flow of oxygen.”
Keeping the Air “Clean”
Currently, carbon dioxide is removed from the air on the ISS using a machine aboard the Zvezda Service Module. This system relies on a material called zeolite, which acts as a molecular sieve, according to Jim Knox, a CO2 control specialist at MSFC.
The CO2 is then vented into space, although engineers are exploring ways to recycle the gas for reuse.
In addition to CO2, astronauts also exhale small amounts of other gases. Methane and carbon dioxide are produced in the intestines, while ammonia is generated from the breakdown of urea in sweat. Other metabolic byproducts, such as acetone, methyl alcohol, and carbon monoxide, are found in both urine and breath.
Activated charcoal filters are the primary method used to remove these chemicals from the air.
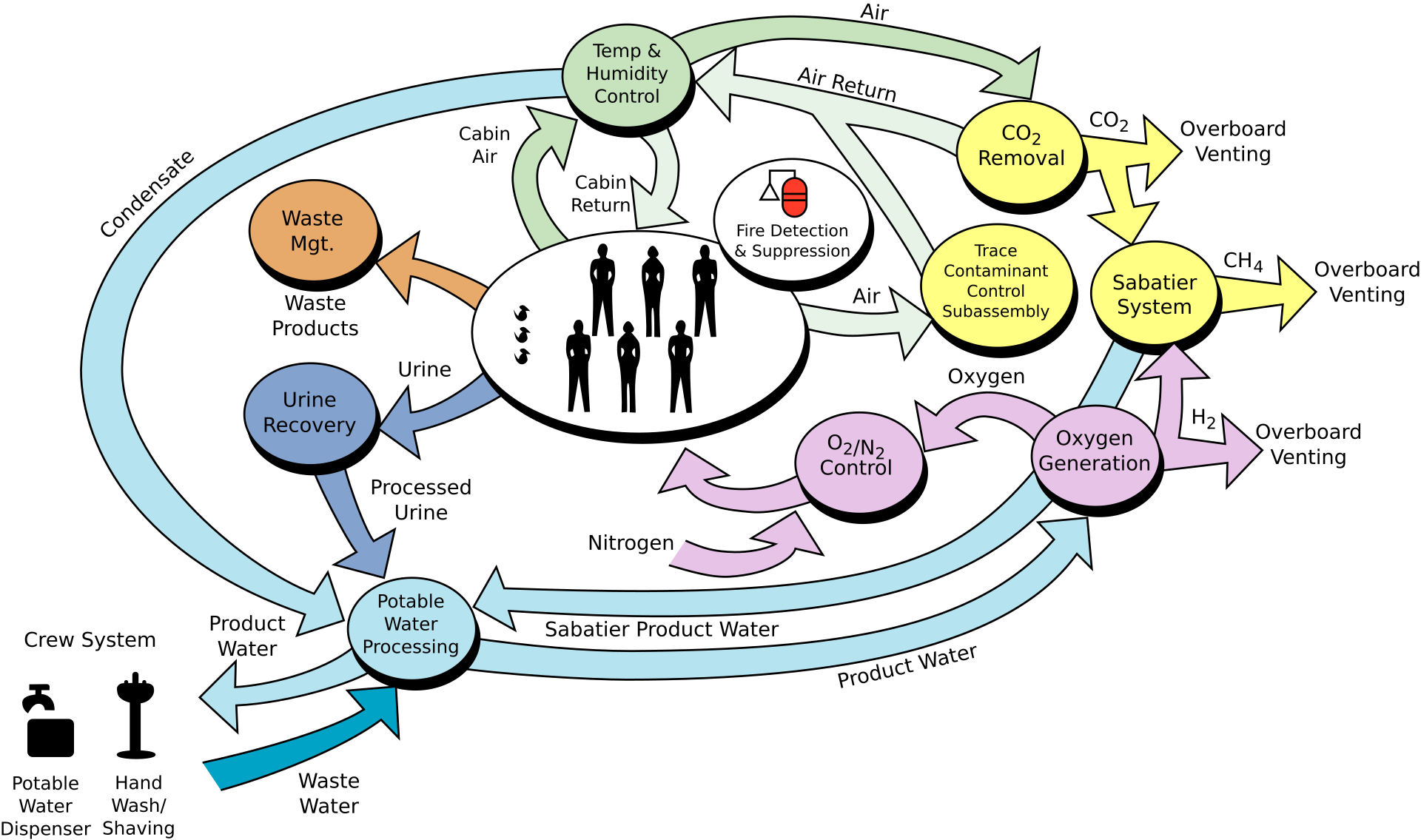
This diagram illustrates the flow of recyclable (“regenerative”) resources within the Space Station’s Environmental Control and Life Support System (ECLSS).
Maintaining a safe and breathable atmosphere aboard the ISS is further complicated by the wide range of chemicals used in the station’s scientific experiments.
“In a 30-year period, there could be numerous types of experimental setups, each requiring a variety of chemical reagents,” Perry explained.
Some of these chemicals could be hazardous, especially if they interact in unexpected ways. Ensuring that these chemicals don’t contaminate the air is critical for the health and safety of the crew.
When the ISS was first being designed, NASA engineers envisioned a centralized system for handling and containing all the chemicals used in experiments. However, this approach proved too complicated.
“The challenge of providing a generic monitoring system to cover the wide variety of chemicals that years of research on the Station will involve—that’s not something the Station itself can easily accommodate,” Perry said.
“It made much more sense for each experimental facility on the lab module to manage its own chemical containment, taking full responsibility for the chemicals from start to finish,” Perry said.
Before each experiment is conducted, a safety review will assess the level of containment required for the rack-mounted facilities. In the event of a chemical release, the crew will seal off the affected module and follow cleanup procedures, if feasible.
However, careful planning and robust hardware design are intended to minimize such risks, ensuring that the crew aboard the Space Station can breathe easily.